Read this blog in:
Soap and alcohol vs the Coronavirus
It has been several months since we have faced with the horror, the novel coronavirus. It was not long after that, the number of cases jumped quickly, and quarantines were implemented by several countries. Now, there are more than 40 million cases worldwide. We have taken several precautions including mask, social distancing but also use of soap and hand sanitizers. But how do those soap and alcohol-based hand sanitizers really work? In this post, I will share with you the science behind the soap and alcohol-based hand sanitizers work mechanism. So, if you are curious, bear with me till the end.
Soap

Surfactant structure
Soaps are made of surfactants and a surfactant is a molecule which has two components, polar and nonpolar. We can also say hydrophilic, water loving, for polar components and hydrophobic, water hating, for nonpolar components. A little bit of science here; the polar components attract polar components like nonpolar attract nonpolar ones. Since surfactants have the both components, they can be used as mediators between these two components which cannot stand to be close to each other. An example is water and oil. if you try to mix them, they will not mix, and they will have this barrier between them due to their polarity. Because water is polar, and oil is nonpolar, thus they are not happy for being together. In order to break that barrier, our lovely mediators (detergents, soaps, basically surfactants) are used. This is the reason why we use soap to clean our hands. In order to rinse the dirt, soil, fat or various organic compounds off easily, we need surfactants to ease the cleaning. And this is also how you remove the things including germs like viruses mechanically. Though water can also remove some germs, it
might not be enough to remove all so a surfactant can increase the efficiency.
But it is not only that, envelop viruses like corona virus have lipid bilayers within their cell membrane, these lipids in their membrane are like surfactants, they have polar and nonpolar parts and they are the thing which keeps the whole structure together and they are used when entering a host cell. Once they come in contact with surfactant, the surfactants can progressively remove these layers as they attract to the polar and nonpolar parts of the surfactant. This results in membrane damage which hold the whole virus together. And all together, it causes leaking in the virus, make the virus not able to enter into a host cell and kill the virus.
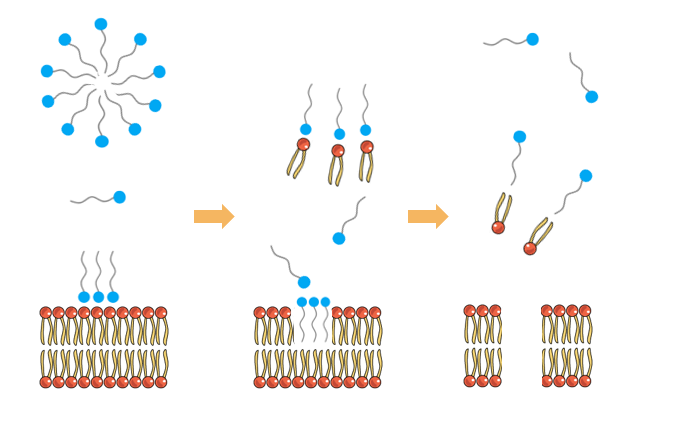
Surfactants removing phospholipids from the cell membrane
Alcohol
Alcohols used as disinfectants are generally ethanol and isopropyl alcohol. They are solvents and a bit like surfactants, they have polar and nonpolar parts. You can mix them with water and you can also mix them with oil. Since alcohol is a solvent, it can dissolve the lipid membrane. Furthermore, it can denature and coagulate the proteins which means changing protein structures in a way that they cannot function same any longer. However, you need high amount of alcohol for it to work. Although smaller amounts can have annihilating effects too, the recommended amount of alcohol is +60%. Too little might not be effective at dissolving and killing the virus.
Additionally, these methods work same for bacteria too, since they have lipid membrane. And interestingly, guess what, human cells have these lipid membranes too. So, it is important not to misuse (drink, eat or inject into the body) these. What about our skin? How is it ok to use them in our body wash? Our skin is one big organism that protects us from environmental factors. Top of our skin consists of dead cells filled with keratin. It is a protein which protects and holds the cells from rupturing when we wash our hands.
Finally, both methods can provide protection and soap can be preferred if there is an access to it since it can remove thing like dirt, soil, fat and make the hands less sticky. However, one needs to be careful not cause any damage to skin. The dryness and further cracks could possibly allow germs to enter the body.
Now you know the science behind! Keep washing and sanitizing your hands. Be safe, healthy and corona free!




